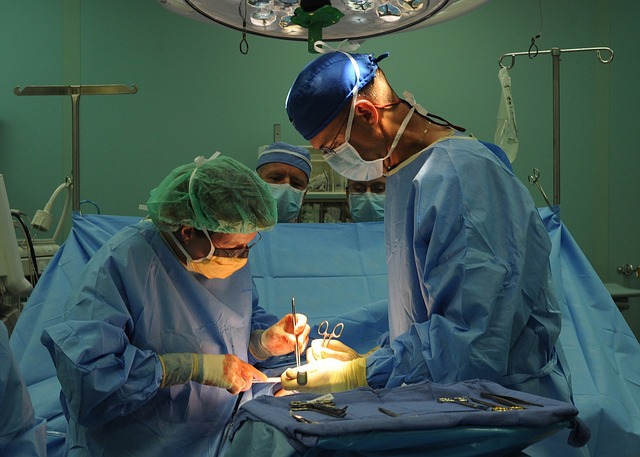
Federal officials warn that over five hundred thousand patients who had open-heart surgery in the United States from 2012 onwards could contract a deadly bacterial infection associated with a device used in the operations.
This year alone, 28 cases have been identified across Iowa, Michigan and Pennsylvania. These infections are rare, but they may cause severe illnesses and at worst, death. They are particularly difficult because they cannot be detected immediately, and may be asymptomatic, or not show any signs until months later.
The device in question is a heater-cooler unit, a piece of medical equipment that keeps a patient’s organs and blood at a certain temperature during surgical procedures. It is used in some 250,000 heart bypass operations annually. Around 60% of these use the model that has been linked to the infections, particularly a bacteria called nontuberculous mycobacterium, or NTM, the Washington Post reports.
NTM occurs naturally, and are not usually harmful. But it can cause infections in patients who have undergone invasive procedures, especially if they have weakened immune systems.
Symptoms include night sweats, weight loss, muscle aches, fatigue and fever, which are all common. These can cause misdiagnosis or delayed diagnoses, resulting in difficult treatment. Medication for the infections is composed of a specific antibiotic regimen to target the slow-growing bacteria.
The Centers for Disease Control and Prevention and the Food and Drug Administration have contacted hospitals and doctors about the possible contamination in recent months. But genetic fingerprinting has pointed to the strongest evidence that the machines in question were those produced in Germany, meaning there could be more cases across the world.
The device is the Stöckert 3T heater-cooler, manufactured by LivaNova PLC, formerly Sorin Group Deutschland GmbH. They have been available in the country since 2006, and the FDA estimates that there are some 2,000 of these specific models in the country.
The equipment does not come in direct contact with the patient, but it has a water reservoir where bacteria can grow. When used, this water can either evaporate or get sprayed into the air, and can enter a patient’s open chest while in surgery.
In US hospitals that have reported infections, the risk remains low, according to Mike Bell, CDC Deputy Director of Health Care Quality Promotion. But the CDC and the FDA both want to raise public awareness among patients and doctors to make sure all steps are taken to find and treat anyone who may have these infections.