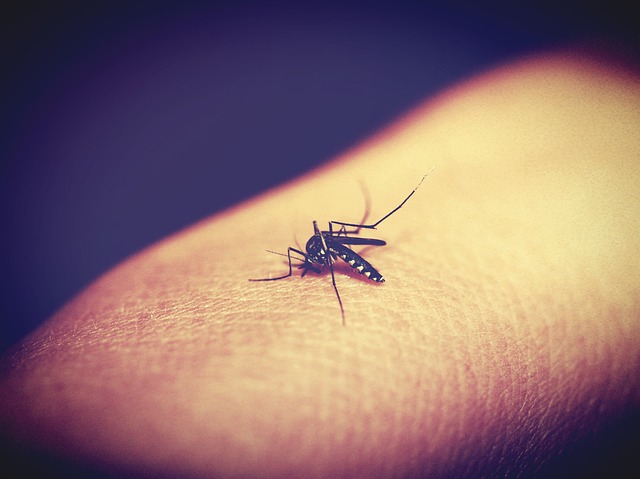
Researchers say that an experimental malaria vaccine seems to prevent the disease in half the people who have tried it for up to a year, NBC reports. This is more effective than the only licensed vaccine currently on the market. The vaccine offers plenty of hope in the fight against malaria, which kills some 500,000 people yearly – most of them children.
Sanaria Inc. in Rockville, Maryland, calls this their first big sunset. A small company that has been operating out of a strip mall, Sanaria makes the vaccine by using the Plasmodium falciparum parasite in mosquitoes. The vaccine was tested on volunteers, and was able to protect 55% of those who took it, stopping the parasite from circulating in their blood in order not to pass malaria on to others.
Dr. Tony Fauci, head of the National Institute for Allergy and Infectious Diseases, says “It is potentially a really very impressive vaccine.” The institute helped test the vaccine.
The testing was in Phase I, which is the primary stage, with just a few volunteers. The vaccine will takes more years of work and research before it is ready for the market.
However, this vaccine’s success rate is higher than the 30% malaria protection offered by GlaxoSmithKilne’s Mosquirix vaccine, which got its approval in Europe last year.
Malaria is caused by a parasite carried by mosquitoes. It can remain in the blood for years. The process of making a vaccine against a parasite is more difficult compared to vaccines for bacteria or viruses, as parasites have complex life cycles that circulate them through the human body.
The Mosquirix vaccine was made using traditional methods: a piece of the protein from the Plasmodium parasite was made into an injection. Sanaria did things differently, by removing the entire parasite in its juvenile state called a sporozite, from mosquitoes. The parasites were radiated then turned into a vaccine.
Dr. Stephen Hoffman, Sanaria’s CEO, says that an intravenous infusion of their vaccine appears to be bringing about positive results.
Our goal is a vaccine that can be used to immunize the entire population to halt transmission and eliminate the parasite. That’s a rather lofty goal.
“Another goal is to able to use the vaccine to prevent malaria in travelers, diplomats, expatriate workers, military personnel; people going in and out of malaria-affected zones,” Hoffman adds.
The NIAID and the University of Maryland recruited 89 healthy people who had never had malaria for the trial. 57 were given the vaccine and 32 were left unvaccinated. All volunteers agreed to be bitten by mosquitoes carrying malaria after three weeks.
Results showed that the vaccine’s effectivity was based on how many doses the recruits were given. Only two out of nine of people who had four doses of the vaccine became infected with the disease, while five out of six unvaccinated volunteers who became ill.
The researchers then tested how long the vaccine would protect the volunteers by dividing them into groups and having them bitten by mosquitoes several more times in the next few weeks.
Five recruits who got four doses never got infected. After 13 months from the first dose, the volunteers were bitten again. None of them got malaria. This was computed at 55% successful.
There were a few side effects found, but blood tests showed that the vaccine was doing well. Other malaria vaccines may prevent you from getting sick from malaria,” Fauci says. “This not only prevents individuals from getting sick, but it prevents them from getting parasites in their blood.”
Hoffman says Sanaria is now beginning tests in Tanzania, Kenya, Mali, Burkina Faso and other countries where malaria has been a consistent problem. “We are not stopping until we get to a defined level of protective efficacy,” Hoffman says.
Hoffman adds that the company wants to be the first to market the world’s first U.S. Food and Drug Administration and European Medicines Agency licensed vaccine. Glaxo’s current vaccine is not FDA-approved.
Sanaria’s funding is coming from the governments of countries where malaria incidences are rampant. “This is an extraordinary change,” Hoffman says. “They want a malaria vaccine that works.”
The study was published in Nature Medicine.